Despite progress in improving treatment regimens for patients with T-cell acute lymphoblastic leukemia (T-ALL), the therapeutic options are still limited, and especially antibody-based immunotherapy is not established. The CD7 antigen represents a promising target structure in T-ALL since it is strongly expressed in different T-ALL subtypes including early T-cell precursor (ETP)-ALL. Therefore, different approaches are currently pursued for targeting CD7, including CAR-T cell therapy. Due to its high internalization capacity CD7 also represents an ideal target structure for antibody drug conjugates (ADC).
Here, a novel antibody engineering approach for CD7-targeting was evaluated in vitro and in xenograft mouse models of T-ALL. A CD7 antibody was optimized for its ability to trigger antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) by introducing two amino acid substitutions (S239D/I332E; DE-variant) in the Fc-domain. In addition, the Fc-engineered antibody was conjugated to the microtubule-disrupting agent monomethyl auristatin E (MMAE) via an enzymatic-cleavable linker (mc-vc-PABC).
The Fc-optimized ADC, CD7-DE-vcMMAE, as well as the unconjugated antibody, CD7-DE, showed improved binding to activating Fcγ receptors (FcγRIIa, FcγRIIIa) compared to a CD7 antibody lacking the DE-modification. This resulted in potent ADCC- and ADCP-activity against different T-ALL cell lines mediated by NK-cells or macrophages as effector cells (EC 50 ADCC, 1-7 pM). In contrast to the CD7-DE antibody, CD7-DE-vcMMAE showed strong cytotoxic effects independently of immune effector cell engagement by releasing its cytotoxic compound into T-ALL cells in an antigen-restricted manner, thereby inducing G2/M cell cycle arrest and apoptosis. CD7-DE-vcMMAE was active at subnanomolar concentrations demonstrating dose-dependent cytotoxic effects in six T-ALL cell lines (IC 50 0.2 - 1 nM). The extent of maximum growth inhibition ranged between 54-98 % and correlated with CD7 antigen density. Yet, although CD7-DE-vcMMAE did not kill CD7-negative cells directly, the linker design facilitated bystander killing activity. Thus, in co-culture experiments using CEM cells and CEM-CD7-knockout cells mimicking CD7-antigen escape, the ADC demonstrated significant killing of neighbouring CD7-negative cells, thereby extending its mode of action.
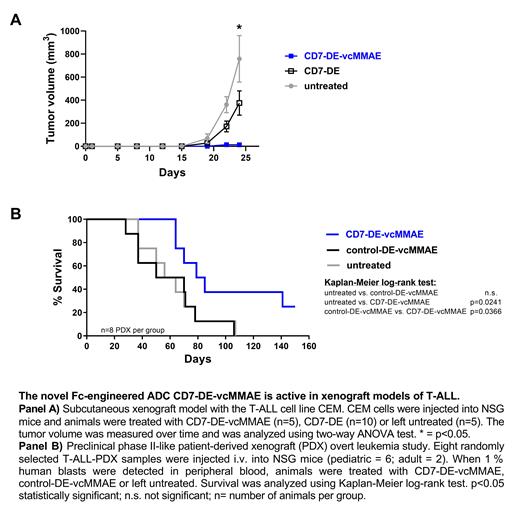
The antitumor activity of the CD7-ADC was further investigated in xenograft mouse models of T-ALL. In a first model, CEM cells were injected subcutaneously into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice and CD7-DE-vcMMAE treatment was evaluated in comparison to the unconjugated antibody CD7-DE. Treatment with CD7-DE-vcMMAE led to a significantly reduced tumor growth in comparison to CD7-DE or untreated animals (p<0.05; see panel A). A preclinical phase II-like patient-derived xenograft (PDX) study employing eight randomly selected T-ALL-PDX samples from pediatric and adult patients was conducted. PDX-cells were injected intravenously into NSG mice and treatment was started when the leukemia load reached 1 % human blasts in the peripheral blood, reflecting an overt leukemia situation. Animals receiving therapy with CD7-DE-vcMMAE showed significant prolongation of median survival in comparison to animals treated with a similarly designed control ADC targeting an irrelevant antigen (control-DE-vcMMAE) or which were left untreated (median survival time; CD7-DE-vc-MMAE: 82 days; untreated/control-DE-vcMMAE: 60 days; p<0.05; see panel B). Importantly, no leukemic blasts were found in the peripheral blood, spleen or bone marrow in animals treated with CD7-DE-vcMMAE and surviving the experimental period of 150 days.
Together, the novel ADC CD7-DE-vcMMAE showed a unique set of Fc effector functions, potent direct growth inhibitory effects, bystander killing activity and efficacy in xenograft models of T-ALL. These results exhibit CD7-DE-vcMMAE as a promising therapeutic strategy and form the basis for new approaches in the treatment of T-ALL.
Disclosures
Cario:Servier, Amgen: Research Funding; JazzPharma: Speakers Bureau. Fransecky:Pfizer: Consultancy; Jazz: Consultancy; Amgen: Consultancy; Gilead: Consultancy, Research Funding, Speakers Bureau; Servier: Consultancy. Baldus:Amgen: Consultancy; Jannsen: Consultancy; AstraZeneca: Consultancy; BMS: Consultancy; Astellas: Consultancy; Jazz Pharmaceuticals: Consultancy; Gilead: Consultancy. Peipp:Merck: Research Funding; Evobright: Consultancy, Research Funding; Biomunex: Consultancy, Research Funding; Janssen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal